Testing for Covid-19 virus. Pic: Oxford University
Covid-19: As Oxford vaccine trials start, experts look ahead to manufacturing at scale
Over a thousand volunteers aged between 18-55 will be receiving a Covid-19 candidate vaccine in trials led by Oxford University. The vaccine was developed in less than twelve weeks by a team led by Sarah Gilbert, professor of vaccinology at Oxford’s Jenner Institute.
The trial programme started on 23 April and will include participants from Oxford, Bristol, Southampton and London. The volunteers will receive either the candidate vaccine or, as part of a control group, a known vaccine for meningitis, which has no efficacy against coronavirus.
The trial vaccine is based on an adenovirus from chimpanzees that has been genetically changed to make it impossible for it to grow in humans. Genes for the spike protein from the surface of the coronavirus have been added to create a vaccine that when injected into a patient starts to produce the spike protein, prompting the immune system to create antibodies. These in turn activate killer T-cells which wipe out the infected cells. If the vaccinated person subsequently gets infected with coronavirus, the immune system triggers the antibodies and T-cells.
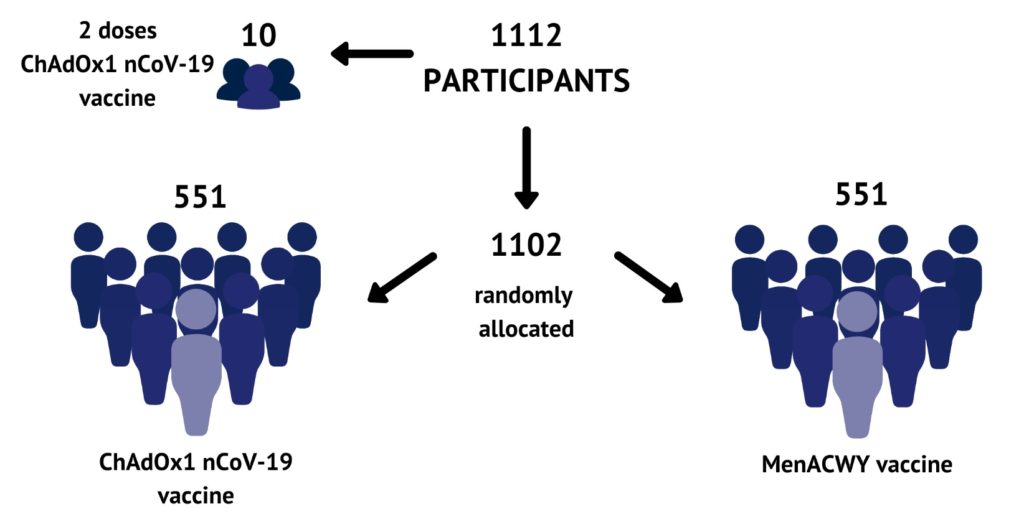
Covid-19 vaccine trial cohorts. Pic: Oxford Vaccine Group at the University of Oxford
Gilbert says that she has a “high degree of confidence in the vaccine but that it must be demonstrated that it works before it can be used in the wider population. This evidence must come from comparisons between the trial cohort and the control group.”
Healthcare workers are being prioritised for the trial, as they are most likely to be exposed to the virus in the coming weeks and months.
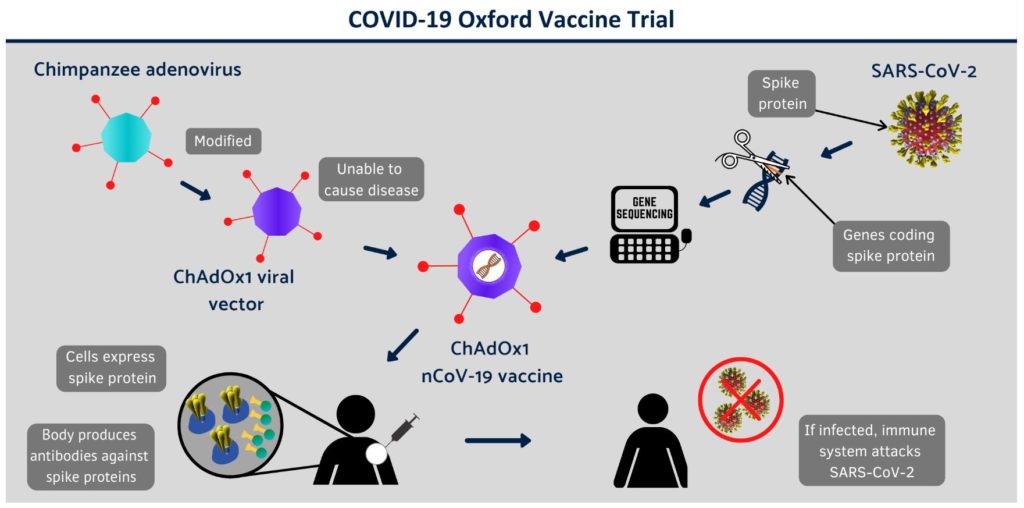
Covid-19 vaccine trial. Pic: Oxford Vaccine Group at the University of Oxford
Statisticians will compare the number of infections in the control group with the number of infections in the vaccinated group and it will be necessary for a small number of participants to develop Covid-19.
How quickly the numbers required are reached will depend on the levels of virus transmission in the community. If transmission remains high, there may be enough data in a couple of months to see if the vaccine works, but if transmission levels drop, this could take up to six months.
If the trials are successful, the next challenge will be to scale up the manufacture to meet a global need for potentially billions of doses. TechTribe Oxford reported earlier this month that construction work on a highly specialist facility that will house the Vaccines Manufacturing and Innovation Centre (VMIC) is being fast tracked at Harwell.
Gilbert explained on BBC1’s The Andrew Marr Show on April 19: “There aren’t any manufacturing facilities in this country that at the moment can make very large amounts of the vaccine. We have a pilot plant in the university that can make small numbers of doses, and that’s what we’re using for the first clinical trials. But we need to go to a much bigger scale. So those companies need to have new equipment, they need to have their staff trained in using new protocols and new quality control assessments. And all of that can happen, but the companies we’re going to be working with will need to stop doing their usual work and make this vaccine instead. So, we need support for them all to make sure that that’s done in a fair way while they’re trying to do something that’s really very important.
“We are looking to protect people’s health, and to do that as widely as possible across the world. It’s not just for this country. We need to make enough vaccine for the world. And there are discussions going on about mechanisms for ensuring fair access to all the vaccines that work at a global level, which we will need to engage with.
“For the moment what we’re concentrating on is getting lots of vaccine available – provided that it does actually work and will protect people. We also want to look at the assessment of other vaccines which are in development because it’s my belief that quite a number of the vaccines in development will work, and they’ll use different manufacturing facilities and that’s another way of getting lots of vaccines available for lots of people.”
